Which Best Describes the Atoms in a Solid
The atoms move closer together and they have more attraction for one another B. Which statement describes all solids.

Atom Neon 3d Model Atom Model Atom Model Project Neon Atom Model
They spread apart as far as possible.

. They are free to move in all directions. They flow with resistance. Atoms in a stay in a fixed position and have no freedom to move.
They are held together by strong attractions. Atoms of Reactant. When a solid element melts which of the following best describes the behavior of atoms.
A piece of solid wax is placed in a pan and heated on a stove. They slide past each other. Cassidy wanted to create an analogy for the motion of atoms in solids liquids and gases so she compared them to marbles in a tray.
They are able to slide past each other. Atoms that once slid past one another now expand in all directions. They have no freedom to move.
They have a definite shape and volume. They are located far apart. The atoms move.
The atoms become larger and they have greater attraction for one another D. Which statement best describes the movement of atoms in a solid. Water has solid and liquid states that do not follow these predictions of.
They are held together by strong attractions. They have a smooth rigid surface. As they move from one carries to another PMF is generated which pumps the H across into the inner.
Which statements describe characteristics of all liquids. C Electrostatic attractions between and - charged ions D Positively charged ions covalently bound with many mobile electrons. Solid whose particles are held together by covalent bonds crystalline solid solid in which the particles are arranged in a definite repeating pattern interstitial sites spaces between the regular particle positions in any array of atoms or ions ionic solid solid composed of positive and negative ions held together by strong electrostatic attractions.
Which statement best describes the atoms in a solid. They are able to slide past each other. They contain loosely packed atoms.
Which statement best describes the atoms in a solid. 3 Which of the following BEST describes the bonding found within solid Al 2 O 3. Which best describes the behavior of atoms in a solid substance that is melting into a liquid.
Which best describes the way particles move to heat objects using conduction. Which one of the following explains why the wax becomes a liquid. They spread to fill available space.
Check all that apply. So To balance the equation put numbers as a prefix to formula unit in equation that make atom of the reactant and product. They slide past each other.
Solids are formed when the forces holding atoms or molecules together are stronger than the energy moving them apart. Atoms that once slid past one another now only vibrate in place. To balance a reaction it is important that the number of reactant atoms must equal to atoms of the product.
Which statement best describes the atoms in a solid. They spread to fill available space. Which best describes the behavior of atoms in a solid substance that is melting into a liquid.
How does the motion of the atoms in the spoon change when the spoon is placed in the hot coffee. Density involves the amount of a material in a certain volume. Liquids have the ability to change shape Liquids flow even.
This module shows how the structure and composition of various solids determine their properties including conductivity solubility density and melting point. Which statement best describes the movement of atoms in a solid. Atoms that were once in fixed positions now slide past one another.
A solid is like the tray being shaken slowly and all the marbles moving in their positions a liquid is like the tray being shaken and the marbles moving around it and a gas is like the tray being shaken hard and the marbles moving vigorously. In a solid the energy transfer mechanisms is occurring between the warmer solid atoms as they collide with each other in what way. After a while the solid wax becomes a liquid.
A Strong covalent bonds between atoms with similar electronegativities B Covalently bound atoms arranged in small individual molecules. They spread apart as far as possible. They are held together by strong attractions.
They have no freedom to move. They are located far apart. When a material changes phases it changes in density in a predictable way as the amount of material stays the same but the molecules get farther apart or closer together.
The module distinguishes the two main categories of solids. FADH2 into the matrix of the mitochondria where they splits from hydrogen atoms and carried by electron carriers. The atoms spread out and they have less attraction for one another C.
They are free to move in all directions. 26 Questions Show answers.

Crystalline Vs Amorphous Solids Animation Solid State Class 11 12 Chemistry Digital Kemistry Youtube Chemistry Covalent Bonding Crystalline Solid

The Solid State Of Matter Chemistry Atoms First

8 5a Atoms Molecules Quiz Quizizz

Chemistry Flip Notes Percent Composition Empirical And Molecular Formulas Molecular Chemistry Formula

Which Part Of An Atom Is Most Directly Involved In Chemical Bonding Ppt Download
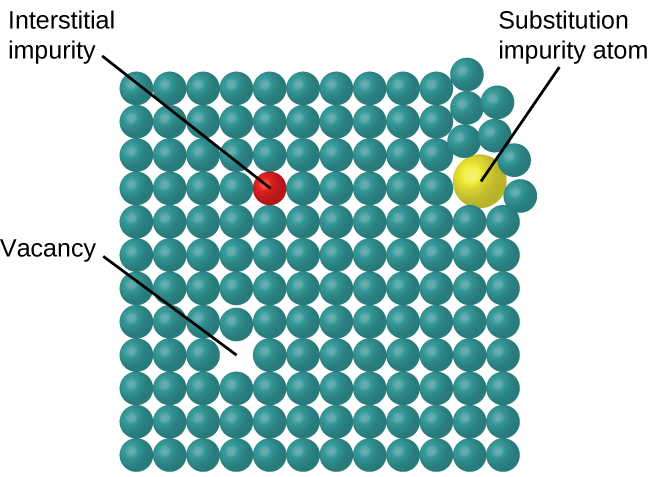
10 5 The Solid State Of Matter Chemistry

Crystal Structure Crystal Structure Metallic Crystal Interactive Lessons

Spin Zone Physicists Get 1st Look At Strange Quantum Magnetism Physicists Holographic Universe Quantum

Atomic Structure Periodic Table Science Quiz Quizizz

Created For An Introduction To Elements Compounds Unit This Worksheet Ca Teaching Middle School Science Middle School Science Resources Chemistry Worksheets

From Wikiwand The Graph Right Shows The Energy Levels As A Function Of The Spacing Between Atoms When The Atoms Are Far Apart R Band Energy Level Band Gap
2 1 Practice Questions Unit 2 Chemistry Quizizz

Atoms And Molecules Interacting With Light Atomic Physics For The Laser Era Hardcover In 2022 Physics Metaphysical Books Molecules

Staar 8 Atoms And Periodic Table Science Quiz Quizizz

Scientists Have Discovered How To Use A New Renewable Energy Source Infrared Radiation Renewable Sources Of Energy Infrared Radiation

Solids Liquids And Gases Elementary Science Matter Science Science Lessons



Comments
Post a Comment